Atomic Structure | Chapter 12 | 8th Science - Student Activities | 8th Science : Chapter 12 : Atomic Structure
Chapter: 8th Science : Chapter 12 : Atomic Structure
Student Activities
Activity 1
Collect more information about the properties of fundamental particles and prepare a chart.
Activity 2
Classify the following ions into monovalent, divalent and trivalent.
Ni2+, Fe3+, Cu2+, Ba2+, Cs+, Zn2+, Cd2+, Hg2+ Pb2+, Mn2+, Fe2+, Co2+, Sr2+, Cr3+, Li+, Ca2+, Al3+
Answer:
Monovalent ions : Li+, Cs+
Divalent ions : Ni2+, Cu2+, Ba2+, Zn2+, Cd2+, Hg2+, Pb2+, Mn2+, Fe2+, Co2+, Ca2+, Sr2 Trivalent ions : Fe3+, Cr3+, Al3+
Activity 3
Write the chemical formula of the compounds
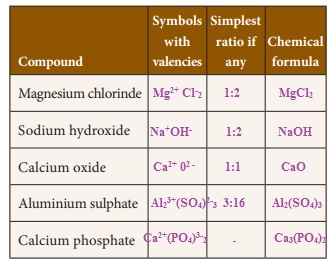
Activity 4
Write the names of the chemical compounds.
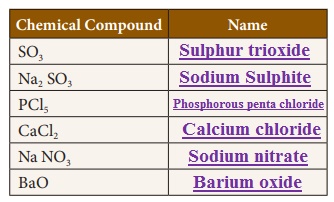
Activity 5
Take some ice cubes in an air tight container and note the weight of the container with ice cubes. Wait for a while for the ice cubes to become water. It is a physical change ie., ice cubes melt and they are converted into liquid. Now weigh the container and compare the weight before and after the melting of ice cubes. It remains the same. Hence it is proved that during a physical change, the total mass of matter remains the same.
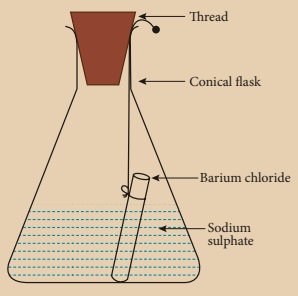
Activity 6
Prepare 5% of barium chloride (5g of BaCl2 in 100 ml of water) and sodium sulphate solutions separately. Take some solution of sodium sulphate in a conical flask and some solution of barium chloride in a test tube. Hang the test tube in the conical flask. Weigh the flask with its contents. Now mix the two solutions by tilting and swirling the flask. Weigh the flask after the chemical reaction is occurred. Record your observation. It can be seen that the weight of the flask and the contents remainsthe same before and after the chemical change. Hence, it is proved that during a chemical change, the total mass of matter remains the same.
John Dalton, son of a poor weaver, began his career as a village school teacher at the age of 12. He became the principal of the school seven years later. In 1793, he moved to Manchester to teach Physics, Chemistry and Mathematics in a college. He proposed his atomic theory in 1803. He carefully recorded each day, the temperature, pressure and amount of rainfall from his youth till the end. He was a meticulous meteorologist.

Electricity, when passes through air, removes the electrons from the gaseous atoms and produces cations. This is called electrical discharge.
The fact that air is a poor conductor of electricity is ablessing in disguise for us. Imagine what would happen if air had been a good conductor of electricity. All of us would have got electrocuted, when a minor spark was produced by accident.
In television tube cathode rays are deflected by magnetic fields. A beam of cathode rays is directed toward a coated screen on the front of thetube, where by varying the magnetic field generated by electromagnetic coils, the beam traces a luminescent image.
When invisible radiation falls on materials like zinc sulphide, they emit a visible light (or glow). These materials are called fluorescent materials.
When hydrogen gas wastaken in a discharge tube, the positively charged particles obtained from the hydrogen gas were called protons. Each of these protons are produced when one electron is removed from one hydrogen atom. Thus, a proton can be defined as an hydrogen ion (H+).
HŌĆā ŌåÆŌĆā H+ + eŌĆō
Related Topics