Biomolecules | Chemistry - Short Answer Questions | 12th Chemistry : UNIT 14 : Biomolecules
Chapter: 12th Chemistry : UNIT 14 : Biomolecules
Short Answer Questions
Biomolecules | Chemistry
Short Answer Questions
1. What type of linkages hold together monomers of DNA?
a)
Hydrogen bonding between complementary base pairs
b)
Base-stacking interactions
2. Give the differences between primary and secondary structure of proteins.
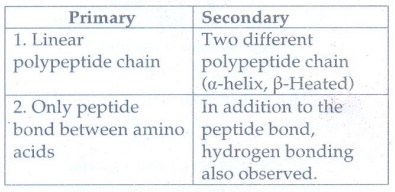
Primary
1.
Linear polypeptide chain
2.
Only peptide bond between amino acids .
Secondary
1.
Two different polypeptide chain (α-helix, β-Heated)
2.
In addition to the peptide bond, hydrogen bonding also observed.
3. Name the Vitamins whose deficiency cause i) rickets ii) scurvy
i)
Rickets - Vitamin D
ii)
Scurvy - Vitamin C
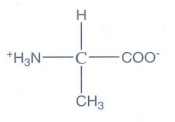
4. Write the Zwitter ion structure of alanine
5. Give any three difference between DNA and RNA
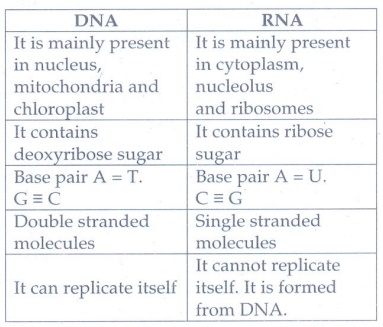
DNA
•
It is mainly present in nucleus, mitochondria and chloroplast
•
It contains deoxyribose sugar
•
Base pair A = T. G ≡ C
•
Double stranded molecules
•
It can replicate itself
RNA
•
It is mainly present in cytoplasm, nucleolus and ribosomes
•
It contains ribose sugar
•
Base pair A = U. C ≡ G
•
Single stranded molecules
•
It cannot replicate itself. It is formed from DNA.
6. Write a short note on peptide bond
The
carboxyl group of the one amino acid react with the amino group of the another
amino acid to give an amide linkage between amino acids. This amide linkage is
called peptide bond.
7. Give two difference between Hormones and vitamins

Hormones
•
It is secreted by tissues in our body
•
It induces a physiological response
Vitamins
•
It cannot be synthesised by our body
•
It is essential for the normal growth and maintenance of our health.
8. Write a note on denaturation of proteins
The
process of a protein-losing its higher order structure without losing the
primary structure, it is called denaturation. When a protein denatures, its
biological function is also lost.
Each
protein has a unique three- dimensional structure formed by interactions such
as disulphide bond, hydrogen bond, hydrophobic and electrostatic interactions.
These
interactions can be disturbed when the protein is exposed to a higher
temperature, certain chemicals such as urea, alteration of pH, ionic strength
etc.,
9. What are reducing and non – reducing sugars?
Reducing sugars
If
the carbonyl group in the sugars are not involved in the glycosidic linkage
then they retain their reducing property such sugars are called non-reducing
sugars.
Eg:
Lactose
Non-reducing sugars
If
the carbonyl group in the sugars are involved in the glycosidic linkage then
they are not available for reduction such sugars are called reducing sugars.
Eg:
Sucrose
10. Why carbohydrates are generally optically active.
Carbohydrates
are having chiral centres. Hence they are optically active
11. Classify the following into monosaccharides, oligosaccharides and polysaccharides. i) Starch ii) fructose iii) sucrose iv) lactose iv) maltose
i)
Starch - Polysaccharides
ii)
fructose - Monosaccharide
iii)
sucrose - Disaccharide
iv)
lactose - Disaccharide
iv)
maltose - Disaccharide
12. How are vitamins classified
i)
Water soluble vitamins. Eg: Vitamin C, B complexes
ii)
Fat soluble vitamins. Eg: Vitamin A, D, E, K.
13. What are harmones? Give examples
Hormone
is an organic substance (e.g. a peptide or a steroid) that is secreted by one
tissue into the blood stream and induces a physiological response in other
tissues. It is an intercellular signalling molecule.
Example:
Insulin, estrogen
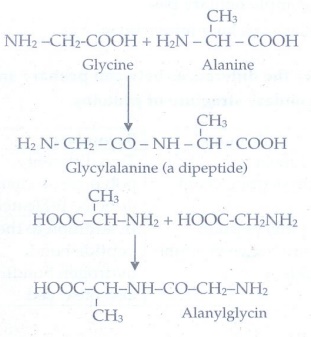
14. Write the structure of all possible dipeptides which can be obtained form glycine and alanine
The
possible dipeptides obtained from glycine and alanine
15. Define enzymes
The
biochemical reactions that occur in our living cells are catalysed by special
proteins called enzymes. They are highly specific in nature.
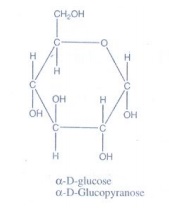
16. Write the structure of α-D (+) glucophyranose
17. What are different types of RNA which are found in cell ?
i.
Ribosomal RNA (rRNA)
ii.
Messenger RNA (mRNA)
iii.
Transfer RNA (tRNA)
18. Write a note on formation of α-helix .
• The amino acids are arranged in a
right handed helical (spiral) structure
• They are stabilised by the
hydrogen bond between the carbonyl oxygen of one amino acid (nth
residue) with amino hydrogen of the fifth residue (n+4th residue).
• The side chains of the residues
project outside of the helix.
• Each turn of an α-helix contains
about 3.6 residues and is about 5.4 Ǻ long.
• The amino acid proline produces a
bend in the helical structure and often called as a helix breaker due to its
rigid cyclic structure.
19. What are the functions of lipids in living organism.
1.
Lipids are the integral component of cell membrane.
2.
Triglycerides is an energy reserve.
3.
They act as protective coating in aquatic organisms.
4.
Lipids of connective tissue give protection to internal organs.
5.
Lipids help in the absorption and transport, of fat soluble vitamins.
6.
They are essential for activation of enzymes such as lipases.
7. Lipids act as emulsifier in fat metabolism.
20. Is the following sugar, D – sugar or L – sugar?
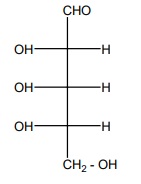
Answer:
L-Sugar
Because
the H and OH on C4 carbon are in the same configuration as the H and
OH on C2 carbon in L-glyceraldehyde.
Related Topics