Matter Around Us | Chapter 9 | 8th Science - Questions Answers | 8th Science : Chapter 9 : Matter Around Us
Chapter: 8th Science : Chapter 9 : Matter Around Us
Questions Answers
TEXTBOOK EXERCISE
I. Choose the best
answer.
1.
The liquid metal used in thermometers is
a) copper
b) mercury
c) silver
d) gold
[Answer: (b) Mercury]
2.
The pictorial symbol for water given by the alchemists was
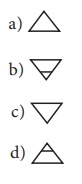
[Answer: (c) ]
3. Which one of the following
element name is not derived from planet?
a) Plutonium
b) Neptunium
c) Uranium
d) Mercury
[Answer: (d) Mercury]
4.
Symbol of mercury is
a) Ag
b) Hg
c) Au
d) Pb
[Answer: (b) Hg]
5.
A form of non-metal which has high ductility is
a) nitrogen
b) oxygen
c) chlorine
d) carbon
[Answer: (d) carbon]
6.
The property which allows the metals to be hammered into their sheets is
___________
a) ductility
b) malleability
c) conductivity
d) shining strength
[ Answer: (b) malleability]
7.
The non-metal which conducts electric current is
a) carbon
b) oxygen
c) aluminium
d) sulphur
[Answer: (a) carbon]
8.
Pencil lead contains
a) graphite
b) diamond
c) aluminium
d) sulphur
[Answer: (a) graphite]
9.
Identify the state of matter based on the arrangement of the molecules.
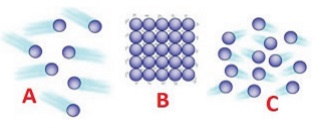
a) A - Gas, B - Solid, C – Liquid
b) A - Liquid, B - Solid, C – Gas
c) A - Gas, B - Solid, C – Liquid
d) A - Liquid, B - Gas, C – Solid
[Answer: (a) A - Gas, B - Solid, C – Liquid]
II. Fill in the
blanks.
1. The element which possesses the character of both metals and non metals are called metalloids.
2. The symbol of tungsten is
3. Melting point of most metal is higher than non-metal.
4. Water contains hydrogen and oxygen element.
5. Silicon / Germanium is used as
semiconductor.
III. Match the
following.
a.
Iron - For making wires
Copper - Sewing needle
Tungsten - As a fuel for ignition in
rocket
Boron - Making the filament of a
bulb
[Answer:
1 - B, 2 - A, 3 - D, 4 – C]
1. Iron (B) Sewing needle
2. Copper (A) For making wires
3. Tungsten (D) Making the filament of a bulb
4. Boron (C) As a fuel for ignition in rocket.
b.
Atom - Building block of matter
Element - Atoms of different kinds
Compound - Atoms of the same kind
Molecule - Smallest unit of a substance
[Answer: (a) 1 - A, 2 - C, 3 - B, 4 - D]
1. Atom (A) building block of matter
2. Element (C) atoms of the same kind
3. Compound (B) atoms of different kinds
4. Molecule (D) smallest unit of a substance
IV. Answer very
briefly.
1.
What is ductility?
Answer: Metals can be drawn into thin wires. This property of metals is called
ductility. Example : Copper wires.
2.
Write the constituent elements and their symbols for the following compounds.
a)
Carbon monoxide b) Washing soda
i) Carbon
monoxide - Compound : (CO), Constituent
elements: Carbon and oxygen
ii)
Washing soda - Compound : (Na2CO3),
Constituent elements: Sodium, carbon and oxygen
3.
Write the symbols for the following elements.
a)
Oxygen b) Gold c) Calcium d) Cadmium e) Iron
(i) Oxygen : O
(ii) Gold : Au
(iii) calcium : Ca
(iv) cadmium : Cd
(v) Iron : Fe
4. Which non-metal is essential for our life
and all living beings?
Answer: Oxygen is essential for our life and all living beings inhale it during
breathing.
5.
Why are bells made of metals?
Answer: On being hit, metals produce a typical sound. They are said to be
sonorous. This property is being made used in making bells.
6.
What does a chemical symbol represent?
Answer: A chemical symbol is a shorthand method of representing an
element.
Symbol of
an element signifies :
(i) Name of the element.
(ii) One atom of the element.
(iii) For example : The symbol O stands for the element of
oxygen.
(iv) One atom of oxygen.
7.
Give two examples for metalloids.
Answer: Boron and silicon.
8.
Mention any three compounds that exist in liquid state.
Answer:
(i) Water
(ii) Hydro chloric Acid
(iii) Nitric Acid
9.
Write three properties of metalloids.
Answer: Properties of metalloids
:
Metalloids are all solid at room temperature.
(i) They can form alloys with other metals
(ii) Some metalloids, such as silicon and germanium, can act as
electrical conductors under the specific conditions, thus they are called
semiconductors.
(iii) Silicon for example appears lustrous, but is not malleable
nor ductile (it is brittle - a characteristic of some non-metals). It is a much
poorer conductor of heat and electricity than the metals
V. Answer briefly.
1.
Can you store pickle in an aluminium utensil? Give reason.
Answer: No, we cannot store the lemon pickle in aluminium utensil because
aluminium is a metal and lemon is acidic. The acids react with metals to give
hydrogen which would spoil the food and makes it unfit to use.
2.
Tabulate the differences between metals and non-metals.
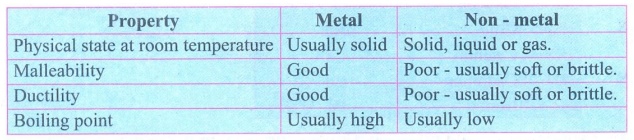
3.
Why are utensils made up of aluminium and brass?
Answer:
(i) The cooking utensils are made up of aluminium and brass
because they are good conductors of heat.
(ii) Aluminium will form a layer of protective oxide that
prevents further reaction. Also aluminium is also relatively cheap and that is
why it is used widely in making utensils.
4.
Define Alchemy.
Answer: Alchemy was form of chemistry studied in the middle age, which was
concerned with trying to discover ways to change ordinary metals into gold.
5.
Name the elements with the following symbols.
a)
Na b) W c) Ba d) Al e) U
Answer:
(i) Na – Sodium
(ii) W-Tungsten
(iii) Ba- Barium
(iv) Al-Aluminium
(v) U- Uranium
6.
Name six common non-metals and write their symbols.
Answer:
Non metals - Symbols
1. Sulphur - S
2. Carbon - C
3. Oxygen - O
4. Hydrogen - H
5. Helium - He
6. Nitrogen - N
7.
Mention any four compounds and their uses.
Answer:
Compounds and their uses :
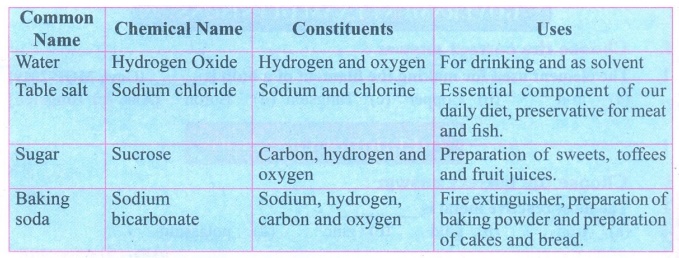
8.
Name the metals that are used in jewellery.
Answer: Silver and gold are used for making jewels and in decorative purposes.
9.
Mention the uses of the following compounds.
a)
Baking soda b) Bleaching powder c) Quick lime
i )
Baking soda : Fire extinguisher,
preparation of baking powder and preparation of cakes and bread.
ii)
Bleaching powder : As bleaching agent,
disinfectant and sterilisation of drinking water.
iii)
Quick lime : Manufacture of cement and
glass.
VI. Given reason.
1.
Give reasons for the following.
a.
Aluminum foils are used to wrap food items.
b.
Immersion rods for heating liquids are made up of metallic substances.
c. Sodium and potassium are stored
in kerosene.
d. Mercury is used in thermometers.
Answer:
(i) Aluminium is malleable, soft and does not react with food
items, so it is used to wrap food items.
(ii) Metals are good conductor of heat and electricity, so
immersion rods are made up of metallic substances.
(iii) Sodium and potassium are very reactive, they react with
air and water, so they are stored in kerosene.
(iv) Mercury is used in thermometers and barometers because of
its high density and uniform expansion at different temperature.
2. Why wires cannot be drawn from
materials such as stone or wood?
Answer: Wires cannot be drawn from materials such as stone or wood, is because these materials are non - metals and are brittle (non - ductile) in nature.
Related Topics