Chemistry - Metallurgy: Choose the correct answer | 12th Chemistry : UNIT 1 : Metallurgy
Chapter: 12th Chemistry : UNIT 1 : Metallurgy
Metallurgy: Choose the correct answer
Choose the correct answer:
1. Bauxite has the composition
a) Al2 O3
b) Al2 O3 .nH2O
c) Fe2 O3
.2H2O
d) None of these
2. Roasting of sulphide
ore gives the gas (A).(A) is a colourless gas. Aqueous solution of (A) is
acidic. The gas (A) is
a) CO2
b) SO3
c) SO2
d) H2S
3. Which one of the
following reaction represents calcinations?
a) 2Zn + O2 →
2ZnO
b) 2ZnS + 3O2
→2ZnO + 2SO2
c) MgCO3 →MgO + CO2
d) Both (a) and (c)
4. The metal oxide which
cannot be reduced to metal by carbon is
a) PbO
b) Al2O3
c) ZnO
d) FeO
5. Which of the metal is
extracted by Hall-Heroult process?
a) Al
b) Ni
c) Cu
d) Zn
6. Which of the
following statements, about the advantage of roasting of sulphide ore before
reduction is not true?
a) ΔGf0
of sulphide is greater than those for CS2 and H2S .
b) ΔG f0
is negative for roasting of sulphide ore to oxide
c) Roasting of the
sulphide to its oxide is thermodynamically feasible.
d) Carbon and hydrogen are suitable reducing agents for metal
sulphides.
7. Match items in column - I with the items of column – II and
assign the correct code.
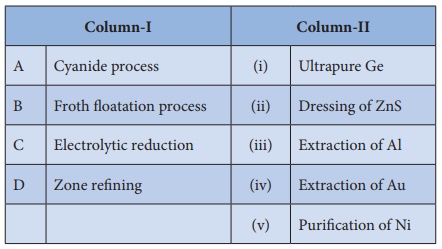
a) A-i , B-ii , C-iii ,
D-iv
b) A-iii , B-iv , C-v ,
D-i
c) A-iv , B-ii , C-iii , D-i
d) A-ii , B-iii , C-i ,
D-v
8. Wolframite ore is
separated from tinstone by the process of
a) Smelting
b) Calcination
c) Roasting
d) Electromagnetic separation
9. Which one of the following is not feasible
a) Zn(s) + Cu2+ (aq) → Cu(s) + Zn2+ (aq)
b) Cu(s) + Zn2+
(aq) → Zn(s) + Cu2+ (aq)
c) Cu(s) + 2Ag+ (aq) → 2Ag(s) + Cu2+ (aq)
d) Fe(s) + Cu2+ (aq) → Cu(s) + Fe2+ (aq)
10. Electrochemical process is used to extract
a) Iron
b) Lead
c) Sodium
d) silver
11. Flux is a substance which
is used to convert
a) Mineral into silicate
b) Infusible impurities to soluble impurities
c) Soluble impurities to
infusible impurities
d) All of these
12. Which one of the
following ores is best concentrated by froth – floatation method?
a) Magnetite
b) Haematite
c) Galena
d) Cassiterite
13. In the extraction of
aluminium from alumina by electrolysis, cryolite is added to
a) Lower the melting point of alumina
b) Remove impurities
from alumina
c) Decrease the
electrical conductivity
d) Increase the rate of
reduction
14. Zinc is obtained
from ZnO by
a) Carbon reduction
b) Reduction using
silver
c) Electrochemical
process
d) Acid leaching
15. Cupellation is a
process used for the refining of
a) Silver
b) Lead
c) Copper
d) iron
16. Extraction of gold
and silver involves leaching with cyanide ion. silver is later recovered by
a) Distillation
b) Zone refining
c) Displacement with zinc
d) liquation
17. Considering
Ellingham diagram, which of the following metals can be used to reduce alumina?
a) Fe
b) Cu
c) Mg
d) Zn
18. The following set of
reactions are used in refining Zirconium
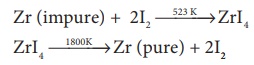
This method is known as
a) Liquation
b) van Arkel process
c) Zone refining
d) Mond’s process
19. Which of the following is used for concentrating ore in
metallurgy?
a) Leaching
b) Roasting
c) Froth floatation
d) Both (a) and (c)
20. The incorrect
statement among the following is
a) Nickel is refined by
Mond’s process
b) Titanium is refined
by Van Arkel’s process
c) Zinc blende is concentrated
by froth floatation
d) In the metallurgy of gold, the metal is leached with dilute
sodium chloride solution
21. In the electrolytic
refining of copper, which one of the following is used as anode?
a) Pure copper
b) Impure copper
c) Carbon rod
d) Platinum electrode
22. Which of the
following plot gives Ellingham diagram
a) ΔSVsT
b) ΔG0Vs T
c) ΔG0 Vs 1/T
d) ΔG0 Vs T2
23. In the Ellingham
diagram, for the formation of carbon monoxide
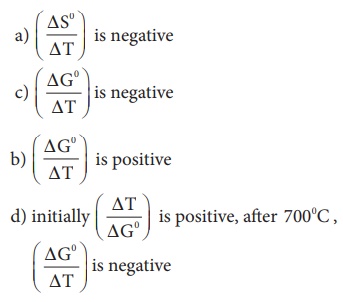
Ans: c) (ΔGº/ΔT) is negative
24. Which of the
following reduction is not thermodynamically feasible?
a) Cr2O3
+ 2Al → Al2O3 + 2Cr
b) Al2O3 + 2Cr → Cr2O3
+ 2Al
c) 3TiO2 +
4Al → 2 Al2O3 + 3Ti
d) none of these
25. Which of the following is not true with respect to Ellingham
diagram?
a) Free energy changes
follow a straight line. Deviation occurs when there is a phase change.
b) The graph for the
formation of CO2 is a straight line almost parallel to free energy
axis.
c) Negative slope of CO shows that it becomes more stable with
increase in temperature.
d) Positive slope of metal oxides shows that their stabilities
decrease with increase in temperature.
Related Topics