Solved Example Problems - Evaluate Yourself: Chemistry: Quantum Mechanical Model of Atom | 11th Chemistry : UNIT 2 : Quantum Mechanical Model of Atom
Chapter: 11th Chemistry : UNIT 2 : Quantum Mechanical Model of Atom
Evaluate Yourself: Chemistry: Quantum Mechanical Model of Atom
Evaluate Yourself
1. Calculate the de-Broglie
wavelength of an electron that has been accelerated from rest through a
potential difference of 1 keV.
Solution:
Given: accelerated potential = 1 keV
The kinetic energy of the electron = the energy due
to accelerating potential.
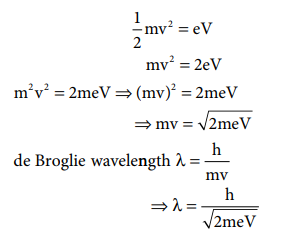
m = mass of the electron = 9.1 ├Ś 10ŌĆō31
kg
h = Planck constant = 6.626 ├Ś 10ŌĆō34 Js 1
1eV = 1.6 ├Ś 10ŌĆō19 J
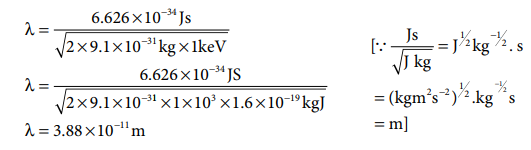
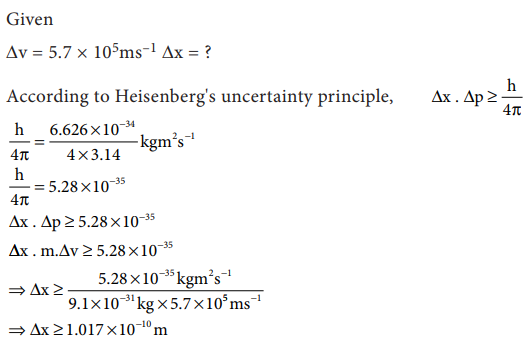
2. Calculate the uncertainty in
the position of an electron, if the uncertainty in its velocity is 5.7 ├Ś 105
ms-1.
Solution:
3. How many orbitals are possible
in the 4th energy level? (n=4)
Solution:
n = 4
l = 0, 1,
2, 3
Ōł┤ 4 sub
shells s, p, d & f.
l = 0 ml = 0 ŌćÆ one 4s orbital.
l = 1 ml = ŌĆō1, 0, + 1 ŌćÆ three 4p
orbitals.
l = 2 ml = ŌĆō2, ŌĆō1, 0, +1, +2 ŌćÆ five 4d
orbitals.
l = 3 ml = ŌĆō3, ŌĆō2, ŌĆō1, 0, +1, +2, +3ŌćÆ
seven 4f orbitals.
Over all 16 orbitals are possible.
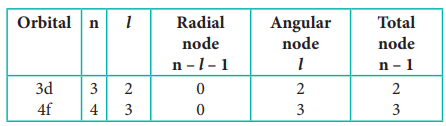
4. Calculate the total number of
angular nodes and radial nodes present in 3d and 4f orbitals
Solution:
5. Energy of an electron in
hydrogen atom in ground state is -13.6 eV. What is the energy of the electron
in the second excited state?
Solution:
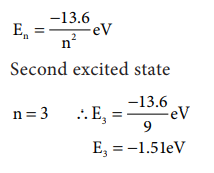
6. How many unpaired electrons are
present in the ground state of Fe3+ (z=26), Mn2+ (z=25)
and argon (z=18)?
Solution:

Electronic configuration of Mn2+ is 1s2
2s2 2p6 3s2 3p6 4s0 3d5
Five
unpaired electrons.
Electronic configuration of Ar: 1s2 2s2
2p6 3s2 3p6
no
unpaired electrons.
7. Explain the meaning of the
symbol 4f2. Write all the four quantum numbers for these electrons.
Solution:
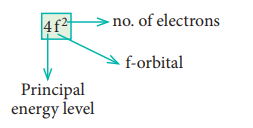
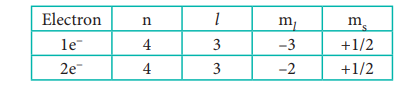
n = 4 ; f orbital l = 3 ŌćÆ ml = ŌĆō 3, ŌĆō2, ŌĆō1, 0, +1, +2
Out of two electrons, one electron occupies 4f
orbital with ml = ŌĆō3 and
another electron occupies 4f orbital with ml
= ŌĆō2.
All the four quantum numbers for the two electrons
are
8. Which has the stable electronic
configuration? Ni2+ or Fe3+.
Solution:
Electronic configuration of Fe3+ : 1s2
2s2 2p6 3s2 3p6 4s0 3d5
Electronic configuration of Ni2+ : 1s2
2s2 2p6 3s2 3p6 4s0 3d8
Fe3+
has stable 3d5 half filled configuration.
Related Topics