Solved Example Problems - Evaluate Yourself: Chemistry: Fundamentals of Organic Chemistry | 11th Chemistry : UNIT 11 : Fundamentals of Organic Chemistry
Chapter: 11th Chemistry : UNIT 11 : Fundamentals of Organic Chemistry
Evaluate Yourself: Chemistry: Fundamentals of Organic Chemistry
Evaluate Yourself
1) Give two examples for each of the following type of organic compounds.
(i) non-benzonoid aromatic,
(ii) aromatic heterocyclic,
(iii) alicyclic and
(iv) aliphatic open chain.
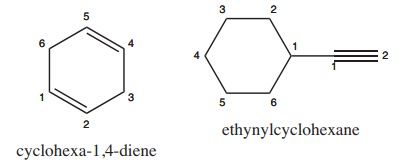
2) Write structural formula for the following compounds
(i) Cyclohexa-1, 4-diene (ii) Ethynyl cyclohexane
Solution:
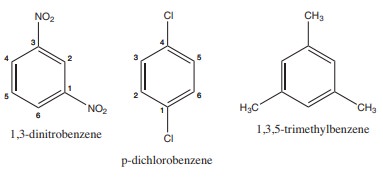
3) Write structural formula for the following compounds
(i) m - dinitrobenzene (ii) p-dichloro benzene (iii)1, 3, 5- Trimethyl benzene
Solution:
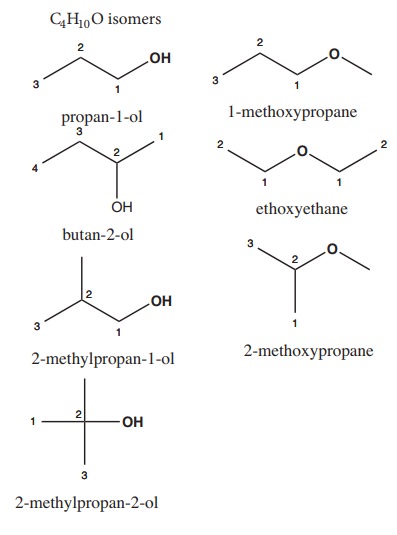
4) Write all the possible isomers of molecular formula C4H10O and identify the isomerisms found in them.
Solution:
5) 0.2346g of an organic compound containing C, H & O, on combustion gives 0.2754g of H2O and 0.4488g CO2. Calculate the % composition of C, H & O in the organic compound [C=52.17, H = 13.04, O = 34.79]
Solution:
Weight of organic substance w = 0.2346 g
Weight of water (x) = 0.2754 g
Weight of CO2 (y) = 0.4488 g
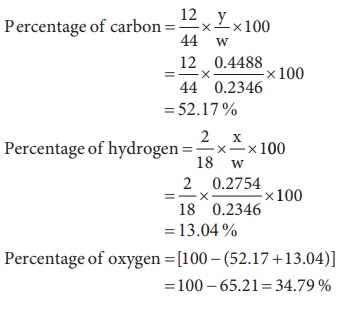
6) 0.16 g of an organic compound was heated in a carius tube and H2SO4 acid formed was precipitated with BaCl2. The mass of BaSO4 was 0.35g. Find the percentage of sulphur [30.04]
Solution:
Weight of organic substance (w)=0.16 g
Weight of Barium sulphate (x) = 0.35 g
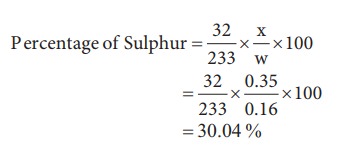
7) 0.185 g of an organic compound when treated with Conc. HNO3 and silver nitrate gave 0.320 g of silver bromide. Calculate the % of bromine in the compound. (Ag =108, Br = 80) Ans: 73.6
Solution:
Weight of organic substance (w)=0.185 g
Weight of silver bromide (x) = 0.320 g
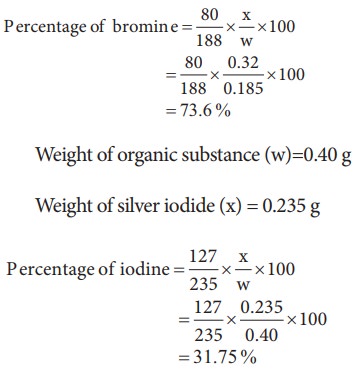
8) 0.40 g of an iodo-substituted organic compound gave 0.235 g of AgI by carius method. Calculate the percentage of iodine in the compound. (Ag = 108 I = 127) (Ans = 31.75)
Solution:
Weight of organic substance (w)=0.33 g
Weight of Mg2P2O7 (x) = 0.397 g

9) 0.33 g of an organic compound containing phosphorous gave 0.397 g of Mg2P2O7 by the analysis. Calculate the percentage of P in the compound (Ans: 23.21) (MFW of Mg2P2O7 is 222 P = 31)
Solution:
Weight of organic compound (w) = 0.3 g
Strength of sulphuric acid used (N) = 0.1 N
Volume of sulphuric acid used (V) = 30 mL
30 ml of 0.1 N sulphuric acid = 30 ml of 0.1 N ammonia
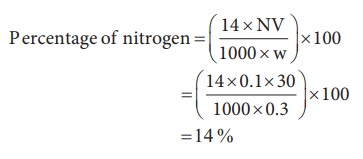
10) 0.3 g of an organic compound on kjeldahl’s analysis gave enough ammonia to just neutralize 30 mL of 0.1N H2SO4. Calculate the percentage of nitrogen in the compound.
Related Topics