Chapter: 11th Chemistry : UNIT 12 : Basic concepts of organic reactions
Brief questions and answers: Chemistry: Basic concepts of organic reactions
Basic concepts of organic reactions | Chemistry
Answer the following questions
16. Write short notes on
a) Resonance
b) Hyperconjucation
(a)
Resonance:
●
Certain organic compounds can be represented by more than one structure and
they differ only in the position of bonding and lone pair of electrons.
●
Such structures are called resonance structures (canonical structures) and this
phenomenon is called resonance. This phenomenon is also called mesomerism or
mesomeric effect.
(b)
Hyperconjugation:
●
The delocalisation of electrons of σ bond is called as hyper conjugation. It is
a special stabilising effect that results due to the Interaction of electrons
of a σ bond (usually C-H or C-C) with the adjacent, empty non-bonding p-orbital
or an anti-bonding σ* or π* orbitals resulting in an extended molecular
orbital.
17. What are electrophiles and nucleophiles? Give suitable examples for each.
Nucleophiles:
●
Nucleophiles are reagents that have high affinity for electro positive centres.
●
They possess an atom with an unshared pair of electrons which can be shared
with the electro positive centre
●
By sharing the electrons they form covalent bonds and gets stabilised.
●
They are usually negatively charged ions or electron rich neutral molecules
(contains one or more lone pair of electrons)
●
All Lewis bases act as nucleophiles.
●
Example : Ammonia, Hydrogen Sulphide, Chlorides, etc.
Electrophiles:
●
Electrophiles are reagents that are attracted towards negative charge or
electron rich center.
●
Neutral molecules like SnCl4 can also act as an electrophile,
as it has vacant d - orbitals which can accomomdate the electrons from Others.
●
They are either positively charged ions or electron deficient neutral
molecules.
●
All Lewis acids act as electrophiles.
Example
: Carbon dioxide, nitroniumion etc.,
18. Show the heterolysis of covalent bond by using curved arrow notation and complete the following equations. Identify the nucleophile is each case.
(i) CH3 - Br + KOH →
(ii) CH3 - OCH3 + HI →
Ans:
i)
CH3 - Br + KOH → CH3 - OH + Br
The
nucleophile here is OH−
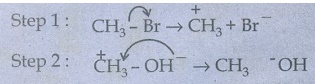
ii)
CH3 - OCH3 + HI → CH3 OH + CH3I
The
nucleophile here is I−
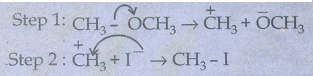
19. Explain inductive effect with suitable example.
Inductive
effect is defined as the change in the polarisation of a covalent bond due to
the presence of adjacent bonds, atoms or groups in the molecule. This is a
permanent phenomenon.
The
inductive effect represents the ability of a particular atom or a group to
either withdraw or donate electron density to the attached carbon.
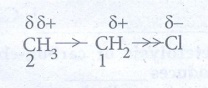
Example:
Ethylchloride.
The
C-C bond in ethyl chloride is polar Chlorine is more electronegative than
carbon and hence it attracts the shared pair of electron between C-Cl in
ethyl chloride towards itself. This develops a slight negative charge on
chlorine and a slight positive charge on carbon to which chlorine is attached.
To compensate it, the C1 draws the shared pair of electron between
itself and C2. This polarisation effect is called inductive effect.
This
effect is greatest for the adjacent bonds, but they also be felt farther away.
However, the magnitude of the charge separation decreases rapidly, as we move
away from C1 and is observed maximum for 2 carbons and almost
insignificant after 4 bonds from the active group.
20. Explain electromeric effect.
Electromeric
effect (E):
Electromeric
effect is a temporary effect which operates in unsaturated compounds
(containing >C=C<, >C=O, etc..) in the presence of an attacking
reagent.
Example:
(i) compounds containing carbonyl group (>C=O) and (ii) unsaturated
compounds such as alkenes (>C=C<)
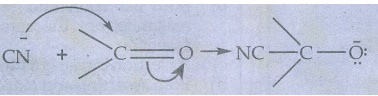
●
When a nucleophile approaches the carbonyl compound, the π electrons between C
and O is instantaneously shifted to the more electronegative oxygen. This makes
the carbon electron deficient and thus facilitating the formation of a new bond
between the incoming nucleophile and the carbonyl carbon atom
(ii)
When an electrophile such as H+ approaches an alkene molecule, the π
electrons are instantaneously shifted to the electrophile and a new bond is
formed between carbon and hydrogen. This makes the other carbon electron
deficient and hence it acquires a positive charge.
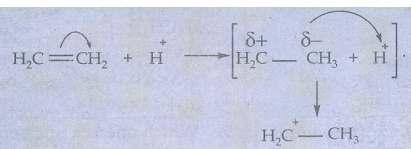
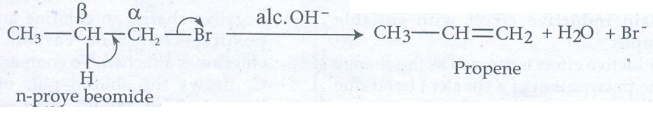
21. Give examples for the following types of organic reactions
(i) β - elimination
(ii) electrophilic substitution.
i) β - elimination:
Example
: n - propyl bromide on reaction with alcoholic KOH gives propene. In this
reaction hydrogen and Br are eliminated.
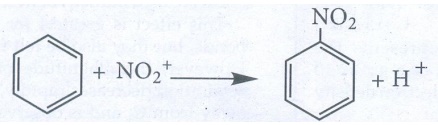
ii) electrophilic substitution : Example : Nitration of
Benzene
Related Topics